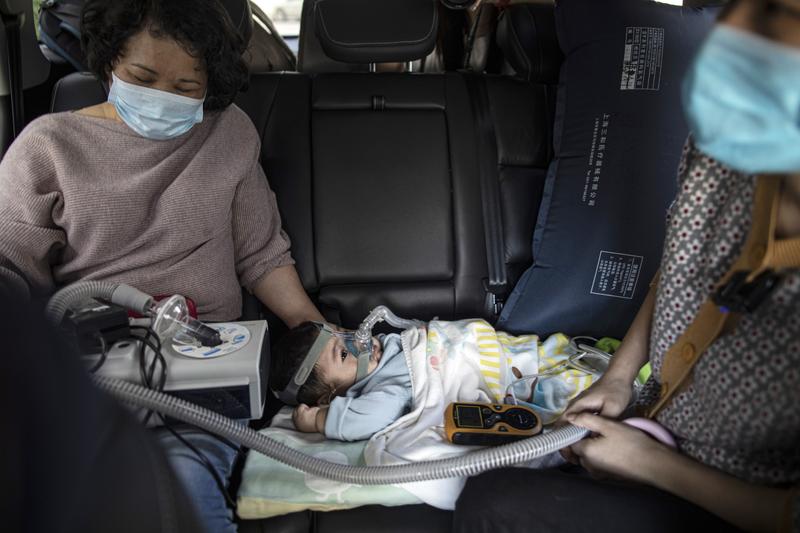
 A 10-month-old patient with spinal muscular atrophy, or SMA, is taken to the hospital in Maoming, Guangdong province, in February. (PHOTO PROVIDED TO CHINA DAILY)
A 10-month-old patient with spinal muscular atrophy, or SMA, is taken to the hospital in Maoming, Guangdong province, in February. (PHOTO PROVIDED TO CHINA DAILY)
Early this month, the world's first therapy to treat spinal muscular atrophy, or SMA, a rare genetic disorder that affects muscle control, was added to the National Reimbursement Drug List in China.
It was the first time that a highly expensive drug to treat a rare condition had been included on the list.
This means that most children who have SMA, which results in death in 95 percent of patients up to 18 months of age, can be saved by an injection at a far more affordable price than previously. Patients who have reached adulthood can also benefit from the therapy.
The treatment was priced at 700,000 yuan ($109,800) per dose when it was launched in China in April 2019. Official data showed that only 50 patients in the country had used it, paying the full price before the therapy was included on the national list. There are estimated to be 20,000 to 30,000 SMA patients in China.
Parents whose children have SMA said they had waited years for affordable medication, adding that it was unbelievable that their dreams had eventually come true.
Xing Huanping, executive director of the Mei'er Advocacy and Support Center for SMA, which has some 2,000 patients registered nationwide, said: "Almost all the parents burst into tears on hearing this news. This disease has put them through the mill, but finally the availability of the therapy, which once had a sky-high price, has offered them a ray of hope.
"The families are grateful to the government not giving up on this small patient community and to the pharmaceutical company's sincerity in providing the world's lowest price for the medication and choosing to stand with them," she said.
In addition to the SMA injection developed by Biogen, which is based in the United States, six other therapies to treat rare diseases have been added to the updated National Reimbursement Drug List, which takes effect next month. They include the world's first and only drug approved to treat walking impairments among patients with multiple sclerosis, which was filed with the National Medical Products Administration this year and given market approval in May.
Feng Jiamei, founder and director-general of the Mei'er center, said, "These past five years have seen tremendous developments in national policies, medical solutions and social awareness of rare diseases.
"With improved drug accessibility and affordability, we believe the plight of patients will be significantly changed. They're expected to survive with a better quality of life and be both a reduced family and economic burden."
Rare diseases have attracted increased public attention.
In 2018, China's first rare disease list, comprising 121 such diseases, was drawn up. A series of measures has also been announced to encourage research into these diseases and the development of orphan drugs-those used to treat rare medical conditions, but which are unprofitable to produce without government assistance.
 Children with rare diseases are taken on a trip in Anhui province in June. (SHI YALEI / FOR CHINA DAILY)
Children with rare diseases are taken on a trip in Anhui province in June. (SHI YALEI / FOR CHINA DAILY)
Destiny changed
According to experts, the Biogen injection benefits SMA patients of all types and at all stages of the disease.
Xiong Hui, deputy director of pediatrics at Peking University First Hospital, said: "In the past, after diagnosis we could only tell parents that we didn't have an effective cure. But now, with such therapies, we can confidently say we can change patients' survival chances and their quality of life.
"Also, medication administered early will always do better. It's hard for those who have already developed muscular atrophy, severe muscle weakness and even spinal joint deformities to run and jump like their peers of the same age," said Xiong, who is also a member of the standing committee of the Beijing Medical Association's rare disease branch.
Clinical trials of Biogen's therapy to treat SMA found that young people showed obvious changes in their physical function, cognitive function and social skills after six months of medication.
Xing said: "A 2-year-old girl with SMA crawled for the first time after undergoing a course of injections for three months."
Mao Jianhua, vice-president of the Children's Hospital at Zhejiang University School of Medicine, said there has long been a severe shortage of doctors able to diagnose rare diseases.
In Shanghai last month, during discussions by experts at an event held by pharmaceutical company Sanofi Genzyme China, Mao said, "When the medication needs of more patients with rare diseases are met, more doctors will have the confidence to learn more about these diseases.
"They will be confident of using a standardized diagnosis and treatment model and jointly helping improve the country's overall ability in rare disease diagnoses and treatment."
Wang Lin, secretary-general of the joint meeting of chairpersons of national rare disease academic groups, said: "The inclusion of rare disease therapies on the National Reimbursement Drug List will help medical insurance play a leading role in the research and development of drugs to treat such diseases. It will also encourage more pharmaceutical companies to conduct R&D on new drugs based on clinical needs."
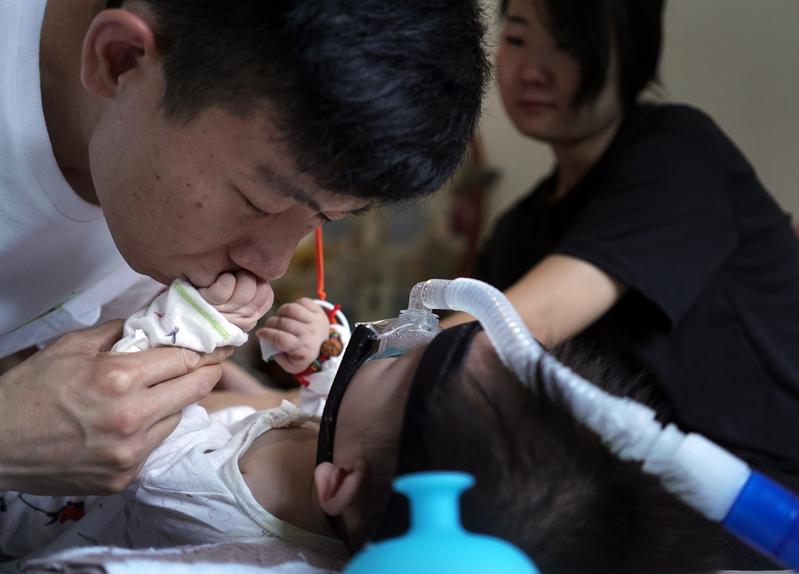 A father in Xiamen, Fujian province, kisses his daughter, who has SMA. (LIU TAO / FOR CHINA DAILY)
A father in Xiamen, Fujian province, kisses his daughter, who has SMA. (LIU TAO / FOR CHINA DAILY)
Regional efforts
Drug accessibility in some regions of China has improved in recent years, with government and commercial medical plans being unveiled.
In March, seven rare disease therapies, including the SMA injection, were included in the medical insurance program in Chengdu, capital of Sichuan province, for significant reimbursement.
Officials from the Chengdu Healthcare Security Administration said about 30 patients have benefited from the city's policy, including Huasheng, a boy diagnosed with SMA last year, when he was 2.
The boy's mother, Li Ling, said, "Such policies have completely changed the destiny of my son and our family." She added that she first took the toddler for medical advice after finding that he could not stand up after falling over when he was 15 months old and that his situation deteriorated.
Li said that after being given four doses of the medication, her son has made continuous progress and can now walk stably, which he could not do before.
"I can sense the changes in him, both physically and mentally. He has become more outgoing after discovering he can make movements he could not perform previously," she said.
Luo Rong, director of the neurology department at West China Women and Children's Hospital in Chengdu, said the city's medical reimbursement program is the result of collaboration by the government, commercial insurance providers and drug developers offering concessions.
Meanwhile, in Guangzhou, capital of Guangdong province, a local government insurance program for patients with rare diseases took effect in January, with medical bills for such patients falling to about 178,000 yuan a year.
 A rare disease patient works as a volunteer in Beijing. (LI MUYI / FOR CHINA DAILY)
A rare disease patient works as a volunteer in Beijing. (LI MUYI / FOR CHINA DAILY)
Schooling difficulties
Thanks to the nation's streamlined medical regulatory mechanism and a system to encourage innovation in meeting medical needs, more than 70 therapies for 60 rare diseases have become available in China due to accelerated drug approvals in recent years.
However, rare disease patients still encounter difficulties such as getting an education.
Li, the mother, said that only one kindergarten had reluctantly agreed to accept her son, but it did not allow the boy's parents to accompany him on campus.
"If we cannot be with him, I will be concerned about his safety. But if he doesn't go to kindergarten, he will have problems with social interaction," she said.
Xing, from the Mei'er patient community, said most young SMA patients do not go to kindergarten or even primary and junior middle school. Various reasons have been cited for this, such as a lack of elevators and barrier-free facilities, along with difficulties in using toilets on campus.
Yin Mei, from Chengdu, whose 6-year-old son has SMA, said, "We really hope that such children can receive an equal education and contribute to society after drugs give them the chance to survive."
In July, the boy, initially predicted by doctors to live for only up to four years and who used to require frequent treatment in intensive care units, received his first therapy thanks to the policy in Chengdu.
"My son is really clever. He could recite dozens of traditional Chinese poems before he was 2 and is also accomplished at math," Yin added.
Yang Hong contributed to this story.


