The World Health Organization announced on Friday it will give emergency use authorization to the COVID-19 vaccine manufactured by China's Sinopharm.
The greenlight for the Sinopharm vaccine to be rolled out globally could pave the way for millions of doses to reach needy countries and boost WHO-backed efforts such as the COVAX initiative, which is a global effort aimed at ensuring access in poorer nations to novel coronavirus vaccines.
The shot’s efficacy for preventing symptomatic and hospitalized disease was estimated to be 79 percent in all age groups combined, the WHO said
The WHO program has already distributed over 54 million doses of COVID-19 vaccines.
READ MORE: WHO: Sinopharm, Sinovac vaccine data show efficacy
The move marks the first time any Chinese-made vaccine has received emergency authorization from the WHO.
During a media briefing, the head of the WHO, Tedros Adhanom Ghebreyesus, said: "WHO gave Emergency Use Listing to Sinopharm Beijing's COVID-19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality."
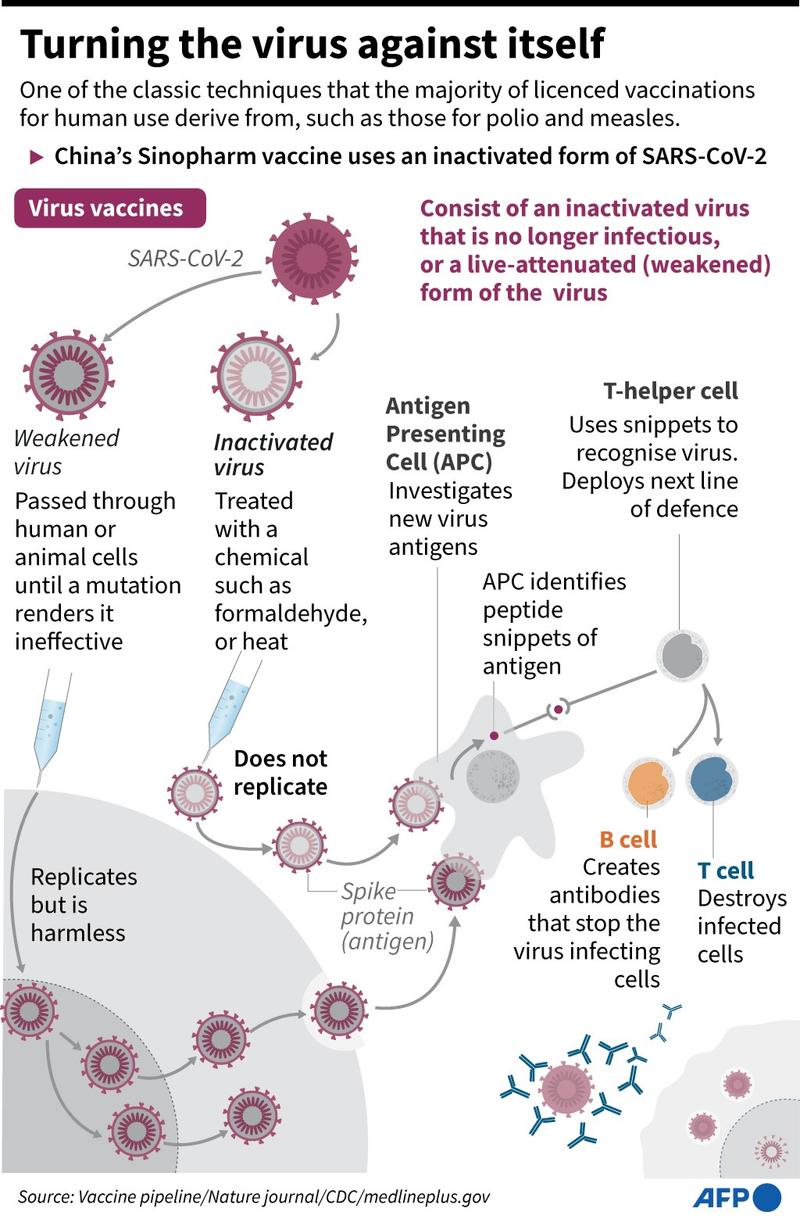
He added: "This expands the list of COVID-19 vaccines that COVAX can buy, and gives countries confidence to expedite their own regulatory approval, and to import and administer a vaccine."
The WHO said it is also the first vaccine that will carry a vaccine vial monitor, a small sticker on the vaccine vials that change color as the vaccine is exposed to heat, letting health workers know whether the vaccine can be safely used
The Sinopharm vaccine joins a list of WHO approved vaccines, in addition to those made by Pfizer, AstraZeneca, Johnson & Johnson and Moderna.
"The addition of this vaccine has the potential to rapidly accelerate COVID-19 vaccine access for countries seeking to protect health workers and populations at risk," said Mariangela Simao, WHO assistant-director general for Access to Health Products. "We urge the manufacturer to participate in the COVAX Facility and contribute to the goal of more equitable vaccine distribution."
ALSO READ: Chinese vaccines rollout picks up steam worldwide
According to the WHO's Strategic Advisory Group of Experts on Immunization (SAGE), the Sinopharm vaccine is recommended for use in adults 18 years and older in a two-dose schedule with a spacing of three to four weeks.
The shot’s efficacy for preventing symptomatic and hospitalized disease was estimated to be 79 percent in all age groups combined, the WHO said.
Though few adults over 60 years were enrolled in clinical trials and efficacy could not be estimated in this age group, the WHO is not recommending an upper age limit for the Sinopharm vaccine, because reviewed data have suggested that the vaccine is likely to have a protective effect in older persons, according to the WHO press release.
"There is no theoretical reason to believe that the vaccine has a different safety profile in older and younger populations," said the WHO press release, which recommends that countries using the vaccine in older age groups conduct safety and effectiveness monitoring.
Experts say the Sinopharm vaccine is easy to store, making it "highly suitable for low-resource settings".
First jab with vial monitor
The WHO said it is also the first vaccine that will carry a vaccine vial monitor, a small sticker on the vaccine vials that change color as the vaccine is exposed to heat, letting health workers know whether the vaccine can be safely used.
 In this Jan 19, 2021 file photo, a medical worker poses with a vial of the Sinopharm's COVID-19 vaccine in Belgrade, Serbia.
(DARKO VOJINOVIC/AP)
In this Jan 19, 2021 file photo, a medical worker poses with a vial of the Sinopharm's COVID-19 vaccine in Belgrade, Serbia.
(DARKO VOJINOVIC/AP)
Assessment by SAGE has shown that the Sinopharm vaccine has been authorized by 45 countries or jurisdictions for use in adults 18 years or older, where more than 65 million doses had been administered through emergency use programs.
Recipients of the vaccine include those in Latin America such as Brazil, Mexico and Chile as well as countries in Asia, including Indonesia, Malaysia and Thailand.
According to Yu Qingming, chairman of the Sinopharm Group, this year's output of the Sinopharm vaccine is targeted at more than 1 billion doses, and is expected to reach 3 billion doses in the future
No safety concerns have been identified from pre-clinical or repro/tox (reproductive toxicity) studies, while most adverse events were mild to moderate, such as injection pain, headache and fatigue.
3 million doses per year
As of Wednesday, more than 1.1 billion COVID-19 vaccine doses had been administered globally, but over 80 percent of those had been administered in high and upper-middle income countries, while just 0.3 percent in low-income countries, according to Tedros.
With the WHO's validation for emergency use, the Sinopharm vaccine, as the first COVID-19 vaccine developed by a non-western country, is expected to accelerate vaccine rollout in many low and middle-income countries through purchase and delivery by the WHO-led COVAX initiative.
According to Yu Qingming, chairman of the Sinopharm Group, this year's output of the Sinopharm vaccine is targeted at more than 1 billion doses, and is expected to reach 3 billion doses in the future.
China has decided to provide ten million COVID-19 vaccine doses to the COVAX initiative to meet the urgent needs of developing countries, a concrete step to deliver on the promise to make vaccines a global public good.
With Xinhua inputs


