State-owned CNBG teams up with partners to step up development of effective, lasting treatment for COVID-19
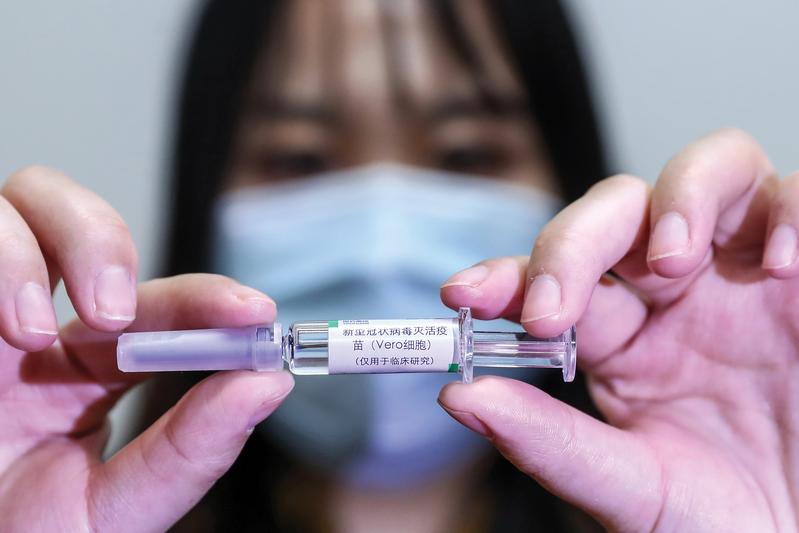 A China National Biotech Group Co Ltd employee displays a COVID-19 inactive vaccine under development and trials at the company’s research institute in Wuhan, Hubei province, on April 10. (ZHANG YUWEI / XINHUA)
A China National Biotech Group Co Ltd employee displays a COVID-19 inactive vaccine under development and trials at the company’s research institute in Wuhan, Hubei province, on April 10. (ZHANG YUWEI / XINHUA)
Chinese State-owned pharmaceutical giant Sinopharm Group has made substantial progress in developing vaccine candidates for the novel coronavirus that causes COVID-19.
China National Biotech Group Co Ltd, Sinopharm’s vaccine and bioscience unit, announced on April 27 that its second inactivated vaccine targeting the contagion won clinical trial approval from the National Medical Products Administration. The vaccine is codeveloped by a unit under CNBG — Beijing Institute of Biological Products Co Ltd — and the Chinese Center for Disease Control and Prevention.
On April 24, CNBG launched phase-2 human trials of its first inactivated vaccine, which is codeveloped by its unit, the Wuhan Institute of Biological Products, along with the Wuhan Institute of Virology under the Chinese Academy of Sciences.
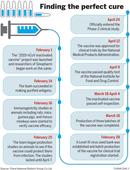
Approved for clinical trials on April 12, the vaccine was the first inactivated vaccine worldwide to reach such a developed approval stage. The NMPA authorized its phase-1 and phase-2 human trials at the same time on that day through a fast-track channel based on solid results from preclinical trial studies.
“The world is racing to develop COVID-19 vaccines, yet it is not a competition between countries, but rather a race between humans and the virus,” said Yang Xiaoming, president of CNBG.
Yang said it is quite encouraging to have developed inactivated vaccines against COVID-19.
Inactivated vaccines use nonliving viruses, bacteria or other pathogens that have lost disease-producing capacity to stimulate the immune system to develop an immune response.
Wang Junzhi, an academic at the Chinese Academy of Engineering, said China has laid a solid foundation to research inactivated vaccines in recent years, and inactivated vaccines have been widely used to fight hepatitis A, influenza, poliomyelitis, and hand, foot and mouth disease.
Yang added that China has distinctive advantages in developing vaccines against the disease thanks to the country’s institutional features that can unite resources and efforts of different participants to concentrate on a specific program and target.
China has adopted five technological approaches to develop COVID-19 vaccines — inactivated vaccines, recombinant protein vaccines, adenovirus vector vaccines, nucleic acid vaccines and vaccines using attenuated influenza viruses as vectors.
The country has now approved one recombinant adenovirus vector vaccine and three inactivated vaccines for clinical trials, while the quest for vaccines using other technical routes is also advancing rapidly.
Apart from Sinopharm’s two inactivated vaccine candidates, an inactivated vaccine developed by Beijing-based Sinovac Research and Development Co Ltd was approved on April 13 for clinical trials.
The recombinant adenovirus vector-based vaccine candidate, developed by the Academy of Military Medical Sciences of the People’s Liberation Army and Chinese firm CanSino Bio, officially entered phase-2 human trials on April 12 and became the world’s first COVID-19 vaccine candidate to reach this stage of trials, said the World Health Organization.
Despite there being no accurate statistics on the exact number of COVID-19 vaccine programs worldwide, the WHO said earlier this month that there were currently 70 vaccine candidates in development.
Sinopharm, officially known as China National Pharmaceutical Group Co Ltd, China’s largest pharmaceutical company with more than 1,500 subsidiaries, including six publicly listed entities, is developing vaccines using two different technological approaches: inactivated and recombinant protein vaccines.
According to a recent report by SinoLink Securities, developing inactivated vaccines usually takes more time compared with newer technologies of nucleic acid and recombinant vaccines because the traditional method has strict R&D conditions including high biosafety standards.
But once successfully synthesized, inactivated vaccines can be massively produced while it is often difficult to produce vaccines using new technologies on a large scale due to a lack of production capacity, the report said.
Liu Jingzhen, chairman of Sinopharm, said the company has set aside a fund of 1 billion yuan (US$141.33 million) to support the vaccines’ R&D.
CNBG’s April 12 randomized, double-blind, placebo parallel-controlled human trials took place in Jiaozuo, Henan province.
The design of the clinical studies is in full accordance with the requirements of national norms, including gradually increasing inoculation dosages and starting with middle-aged groups, said Sinopharm.
As of April 23, a total of 96 people in three age groups aged between 18 and 60 had been inoculated, and all were reported to be in good condition, the company said, adding that participants will continue to be under close observation to assess the vaccine’s safety.
The phase-2 study aims at not only testing the vaccine’s safety, but also figuring out vaccination procedures, including how many doses and how much of each are required.
The vaccine will also go through the third phase of clinical trials, which will mainly test efficacy and safety, said the company.
It may take more than a year to finish all three phases of human trials to determine whether the vaccine is safe and effective in protecting people from COVID-19, the company said.
Thanks to the huge production capacity of units like CNBG, Sinopharm is able to produce 100 million vials of inactivated COVID-19 vaccines per year, it said.
Shi Lichen, founder of Beijing Dingchen Consultancy, said: “The company has demonstrated high-level R&D capabilities as well as a strong sense of corporate social responsibility. It is not a surprise that CNBG has made such achievements due to its strong competence in the vaccine sector, yet developing a new vaccine against the novel coronavirus requires not only strong technical capabilities but also huge amounts of investment and is very risky financially.”
Apart from vaccine research, Sinopharm also has a strong presence in the development of COVID-19 tests and treatments.
Shanghai Geneodx Biotech Co Ltd, an affiliate of CNBG, is one of the first three companies in China to succeed at developing test kits for the novel coronavirus.
CNBG is also the first in China to develop convalescent plasma treatment for patients suffering from COVID-19 using blood plasma taken from convalescents, which is recommended in official guidelines for treatment of the disease.
Sinopharm ranks 169th on the Fortune Global 500 last year, and fourth among pharmaceutical companies.