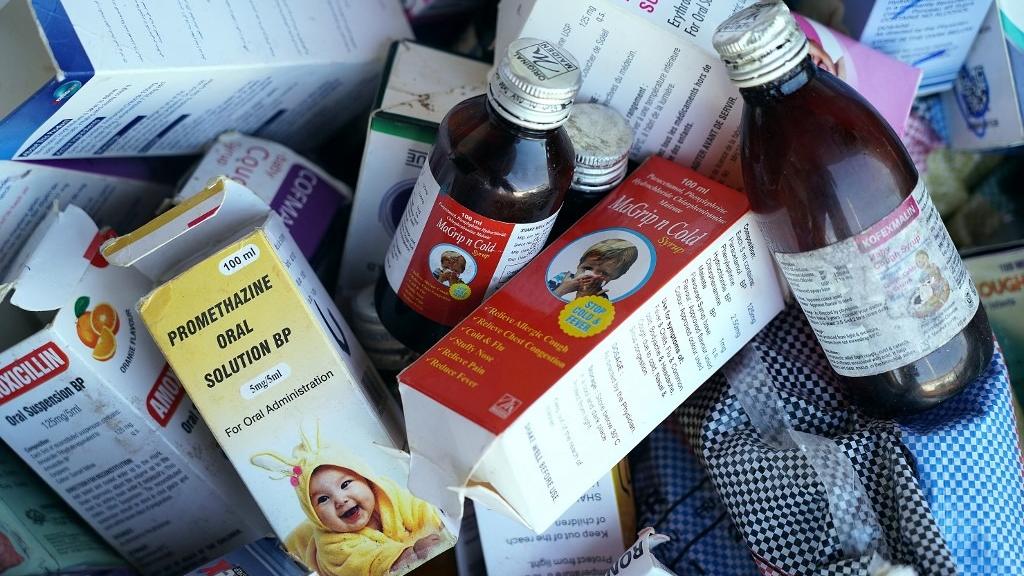
 A photograph shows collected cough syrups in Banjul, Gambia on Oct 6, 2022. (PHOTO / AFP)
A photograph shows collected cough syrups in Banjul, Gambia on Oct 6, 2022. (PHOTO / AFP)
NEW DELHI - India is considering a change to its pharmaceutical industry policy after cough syrups made in the country were linked to the deaths of children overseas, according to a document from Prime Minister Narendra Modi's office, which noted that "important things" about the industry had been "overlooked".
A brainstorming session was held in the southern Indian city of Hyderabad "to find a solution to exported cough syrups that killed children," Modi's office said in the document dated May 15 and reviewed by Reuters.
Health Minister Mansukh Mandaviya and federal and state regulators attended the session in February, according to a statement from the health ministry that did not mention cough syrups.
"Tweak in policy is mooted," the document from the prime minister's office said, adding that "important things" had been "overlooked". It did not elaborate.
A source with knowledge of the matter said the policy change could mean increased oversight of India's $41 billion pharmaceutical industry, which is the world's largest supplier of generic medicines
A source with knowledge of the matter said the policy change could mean increased oversight of India's $41 billion pharmaceutical industry, which is the world's largest supplier of generic medicines.
Increased testing of cough syrups as well as of raw materials for drugs in general is one of the steps being considered, said the source.
The statement, which has not been previously reported, appears to be the first time the prime minister's office has addressed the cough syrup controversy. Modi's office and the health ministry did not respond to a request for comment.
India's drug regulator, the Central Drugs Standard Control Organisation, has proposed testing cough syrups in government laboratories before they are exported, media outlet News18.com reported on Tuesday.
The World Health Organization found last year that cough syrups made by an Indian drugmaker contained dangerous levels of two known toxins, diethylene glycol and ethylene glycol, leading to the deaths of at least 70 children in Gambia. India denies a connection between the syrups and the deaths.
READ MORE: Report: India considering testing cough syrups before export
The WHO says it is still seeking the culprit within the supply chain, but has been frustrated in its efforts, Reuters reported earlier this month.
Indian health officials have expressed concern that the incidents of contaminated syrups will harm its pharmaceutical industry. An Indian representative participated in a meeting of global drug regulators in Indonesia earlier this month to discuss ways to ensure the safety of the pharmaceutical supply chain
India has acted against a second Indian company whose cough syrups were linked to the deaths of 19 kids in Uzbekistan, including the arrest of three of its employees. A third Indian drugmaker was found by the WHO to have sold tainted syrups to the Marshall Islands and Micronesia.
Indian health officials have expressed concern that the incidents of contaminated syrups will harm its pharmaceutical industry. An Indian representative participated in a meeting of global drug regulators in Indonesia earlier this month to discuss ways to ensure the safety of the pharmaceutical supply chain.
"The participating authorities expressed their deep commitment for immediate, short, medium, and long-term actions to strengthen the regulatory systems in safeguarding patients from contamination in medicines," a WHO spokesperson told Reuters.
According to an advisory sent by Indian regulator CDSCO to all states on April 21 and seen by Reuters, drug officials must ensure that drugs meet the standards of the Indian Pharmacopoeia.
ALSO READ: Rash of child deaths in Gambia 'linked to syrups made in India'
The CDSCO said it was reiterating the need for standards after receiving a public complaint about the standards of drug ingredients in the country. It did not say who filed the complaint.
If a drug is not included in the IP, "the standards of identity, purity and strength specified for drugs in the current edition of Pharmacopoeia of any other country ... are applicable and such standards as may be prescribed, shall be followed."
"It is once again requested to ensure compliance with the said standards," said the advisory from Rajeev Singh Raghuvanshi, the drugs controller general of India.