 This Nov 9, 2020 file photo shows a general view of Pfizer Manufacturing Belgium in Puurs, Belgium. (VIRGINIA MAYO / AP)
This Nov 9, 2020 file photo shows a general view of Pfizer Manufacturing Belgium in Puurs, Belgium. (VIRGINIA MAYO / AP)
LONDON - Britain approved Pfizer’s COVID-19 vaccine on Wednesday, jumping ahead of the United States and Europe to formally endorse a jab it said should reach the most vulnerable people early next week.
The shot will be available in the UK from next week, according to a government statement
British Prime Minister Boris Johnson touted the medicine authority's approval as a global win and a ray of hope amid the gloom of the novel coronavirus which has killed nearly 1.5 million people globally, hammered the world economy and upended normal life.
The emergency authorization clears the way for the deployment of a vaccine that Pfizer and its German partner BioNTech have said is 95 percent effective in preventing illness. The shot will be available in the UK from next week, according to a government statement Wednesday.
"It's fantastic," Johnson said. "The vaccine will begin to be made available across the UK from next week. It's the protection of vaccines that will ultimately allow us to reclaim our lives and get the economy moving again."
ALSO READ: Pfizer, BioNTech plan filing as vaccine proves 95% effective
British Health Secretary Matt Hancock said he was "very proud that the UK is the first place in the world to have a clinically authorized vaccine".
“We can see the way out, and we can see that by the spring we are going to be through this,” Hancock said on Sky News. In a radio interview he added that 800,000 doses are ready to be delivered from Belgium. “This is going to be one of the biggest civilian projects in history,” he said, with 50 hospitals preparing to administer the vaccine.
 This undated photo released by Pfizer on Dec 1, 2020 shows a vial of the COVID-19 candidate vaccine developed by BioNTech and Pfizer displayed at a manufacturing plant in Puurs, Belgium. (PHOTO / PFIZER VIA AP)
This undated photo released by Pfizer on Dec 1, 2020 shows a vial of the COVID-19 candidate vaccine developed by BioNTech and Pfizer displayed at a manufacturing plant in Puurs, Belgium. (PHOTO / PFIZER VIA AP)
BioNTech American depositary receipts rose 8 percent in early trading in Germany.
The UK had signaled it would move swiftly in approving a vaccine, and doctors across the country were put on standby for a possible rollout. For the government, it’s an opportunity to make up for missteps during the pandemic as UK's death toll nears 60,000.
The UK's Medicines and Healthcare Products Regulatory Agency said that the vaccine “met its strict standards of safety, quality and effectiveness”
The UK regulator, the Medicines and Healthcare Products Regulatory Agency (MHRA), said on Wednesday that the vaccine “met its strict standards of safety, quality and effectiveness”.
The MHRA approved the vaccine in record time - partly by doing a "rolling" concurrent analysis of data and the manufacturing process while Pfizer raced to conclude trials.
EU application
BioNTech is awaiting a decision from the US Food and Drug Administration in the middle of December, the same time horizon in which it expects a ruling from European Union regulators, Chief Medical Officer Ozlem Tureci said in a press conference.
The German company and its US partner earlier this week sought regulatory clearance in the EU, while an FDA panel is set to meet on Dec 10 to discuss the vaccine.
READ MORE: Moderna, Pfizer-BioNTech seek EU approval for vaccines
China has given authorization to its three front-runners for emergency use. Russia cleared a vaccine known as Sputnik V in August, while a second inoculation was approved in October, even as the last stage of trials to establish safety and efficacy are still taking place.
The British government in late November invoked a special rule allowing its drug regulator to move ahead of the EU as the country prepares for the Brexit transition period to conclude at the end of this year.
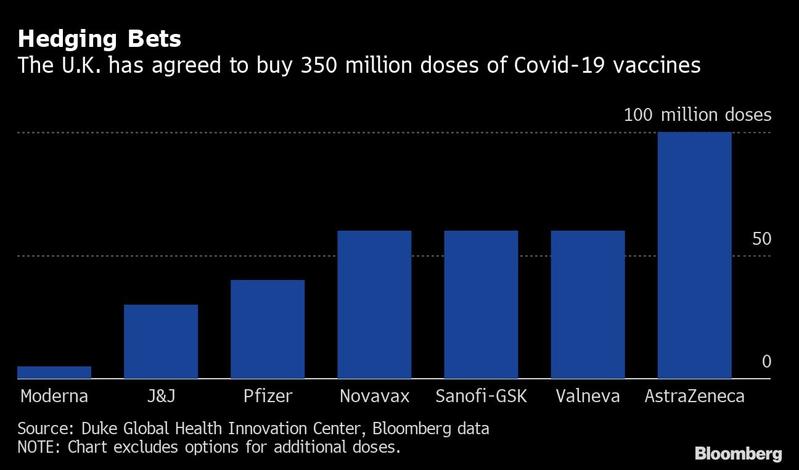
The UK still needs other vaccines to reach the finish line in order to immunize enough of its population to end the pandemic. The country has ordered enough doses of the two-shot Pfizer-BioNTech shot to inncoculate 20 million people, less than one-third of the population. While the companies have said they can produce 1.3 billion doses next year, much of that supply is already spoken for in deals to ship hundreds of millions of shots to Europe, the US, Japan and elsewhere.
'Historic moment'
The approval also marks the first time a vaccine based on messenger RNA has reached the market. The new technology essentially transforms the body’s cells into tiny vaccine-making machines, instructing cells to make copies of the coronavirus spike protein, which stimulates the production of protective antibodies.
Pfizer said Britain's emergency use authorization marks a historic moment in the fight against COVID-19.
The UK has ordered enough doses of the two-shot Pfizer-BioNTech shot to inncoculate 20 million people, less than one-third of the population
"This authorization is a goal we have been working toward since we first declared that science will win, and we applaud the MHRA for their ability to conduct a careful assessment and take timely action to help protect the people of the UK," said CEO Albert Bourla.
"As we anticipate further authorisations and approvals, we are focused on moving with the same level of urgency to safely supply a high-quality vaccine around the world."
First in line?
The UK's vaccine committee will decide which priority groups will get the jab first: care home residents, health and care staff, the elderly and people who are clinically extremely vulnerable will be first in line.
Hancock said hospitals were ready to receive the shots and vaccination centers would be set up across the country but he admitted distribution would be a challenge given that the vaccine must be shipped and stored at -70C, the sort of temperature typical of an Antarctic winter.
READ MORE: Pfizer vaccine results leave questions about safety, longevity
Pfizer has said it can be stored for up to five days at standard refrigerator temperatures, or for up to 15 days in a thermal shipping box.
The Pfizer-BioNTech shot dashed to the head of the queue after delays to the trials of the AstraZeneca-Oxford vaccine, which has also shown promising signs in preliminary results of broad studies. The UK partners have faced questions after acknowledging that a lower dosage level that appeared more effective resulted from a manufacturing discrepancy.


