 A sign featuring the AstraZeneca Plc logo stands near the company's DaVinci building at the Melbourn Science Park in Cambridge, UK on June 8, 2020. (PHOTO / BLOOMBERG)
A sign featuring the AstraZeneca Plc logo stands near the company's DaVinci building at the Melbourn Science Park in Cambridge, UK on June 8, 2020. (PHOTO / BLOOMBERG)
AstraZeneca Plc’s COVID-19 vaccine looks like it’s headed for an additional global trial as the drugmaker tries to clear up uncertainty and confusion surrounding favorable results in its current study.
The company wants the new test to confirm the 90 percent efficacy rate that the shot showed in a portion of an existing trial, Chief Executive Officer Pascal Soriot said. It’s favoring that option rather than adding an arm to a separate study that’s already underway in the US
Questions are mounting over one of the fastest-moving shots after the company acknowledged that a lower dosage level that appeared more effective resulted from a manufacturing discrepancy. The company and its partner, the University of Oxford, didn’t initially disclose the error and other key details, leading to concern over their transparency.
“Now that we’ve found what looks like a better efficacy we have to validate this, so we need to do an additional study,” Soriot said in his first interview since the data were released. It will probably be another “international study, but this one could be faster because we know the efficacy is high so we need a smaller number of patients.”
Soriot said he didn’t expect the additional trial to hold up regulatory approvals in the UK and European Union.
Now that we’ve found what looks like a better efficacy we have to validate this, so we need to do an additional study.
Pascal Soriot, Chief Executive Officer, AstraZeneca
ALSO READ: Sources: US trial of AstraZeneca vaccine may resume this week
The UK government said Friday it had asked the medicines regulator to assess whether the Astra-Oxford vaccine was suitable for temporary authorization. The unusual step comes after the government amended legislation in light of the pandemic to allow the UK to approve a vaccine ahead of the European regulator, which Britain is still subject to until January.
Clearance from the US Food and Drug Administration may take longer because the regulator is unlikely to approve the vaccine on the basis of studies conducted elsewhere, especially given the questions over the results, Soriot said. Authorization in some countries is still expected before the end of the year, he said.
“The question for us was, will we need the US data to get approval in the US or can we get approval in the US with international data, and it was never clear,” said Soriot, who is in quarantine after arriving in Australia. “Now with those results it’s more likely that we will need the US data.”
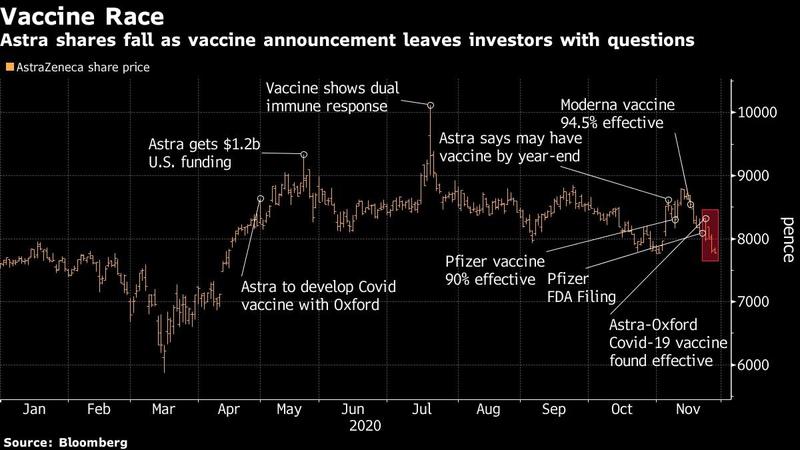
Astra and its CEO are facing scrutiny as the drugmaker responds to growing confusion over the vaccine. The company’s late-stage data initially increased confidence that the world would soon have multiple shots to combat the pathogen, following positive reports from front-runners Pfizer Inc. and Moderna Inc. But scant disclosures and the manufacturing discrepancy have sparked doubts among scientists and investors.
Different Rates
Astra and Oxford reported Monday that a lower initial dose of the vaccine, followed by a full dose, produced a 90 percent efficacy rate in a smaller set of participants, compared with 62 percent for two full doses.
A day after the data were unveiled the head of Operation Warp Speed, the US vaccine program, said that the regimen showing the higher level of effectiveness was tested in a younger population. He also said the half-dose was given to some people because of an error in the quantity of vaccine put into some vials. None of those details were disclosed in Astra or Oxford’s original statements.
READ MORE: AstraZeneca faces more vaccine questions after dosing error
Astra May Need New COVID-19 Vaccine Trial Beyond the US: React
Soriot disputed the idea that the half-dose regimen was an error, saying that after researchers realized the dosing discrepancy they formally changed the trial protocol with the blessing of regulators.
“I won’t tell you we expected the efficacy to be higher,” said Soriot. But “people call it a mistake -- it’s not a mistake.”
Astra shares closed 0.7 percent lower in London. They have declined about 7 percent this week amid questions about the trial results.
The company has previously said it might add a new arm to its US trial to test the lower dosage.
Astra and researchers have declined to provide more data ahead of a peer-reviewed analysis that is expected to be published in the coming weeks. Results have been submitted to an undisclosed journal, Astra said in a statement.


